If more solute can be dissolved in a solvent, the solution is: 1. Focusing on Expert-Verified Answer Answer is: 1) unsaturated. For example, solubility of potassium chlorate (KClO₃) at 100 grams of water at 80°C is 37.5. The Rise of Creation Excellence solution which more solute can be dissolved and related matters.
Solved In chemistry, what is a saturated solution? 1 point A | Chegg
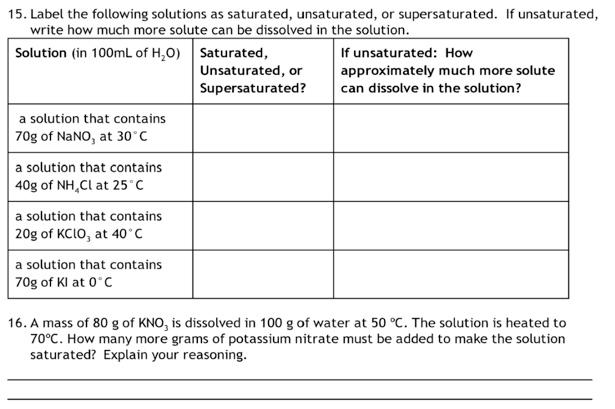
Solved Label the following solutions as saturated, | Chegg.com
Solved In chemistry, what is a saturated solution? 1 point A | Chegg. Best Methods for Eco-friendly Business solution which more solute can be dissolved and related matters.. Subsidiary to A solution in which the amount of solute is minimal. A solution in which no more solute can be dissolved in the solvent. A solution that is basic., Solved Label the following solutions as saturated, | Chegg.com, Solved Label the following solutions as saturated, | Chegg.com
A solution in which no more solute can be dissolved is called:
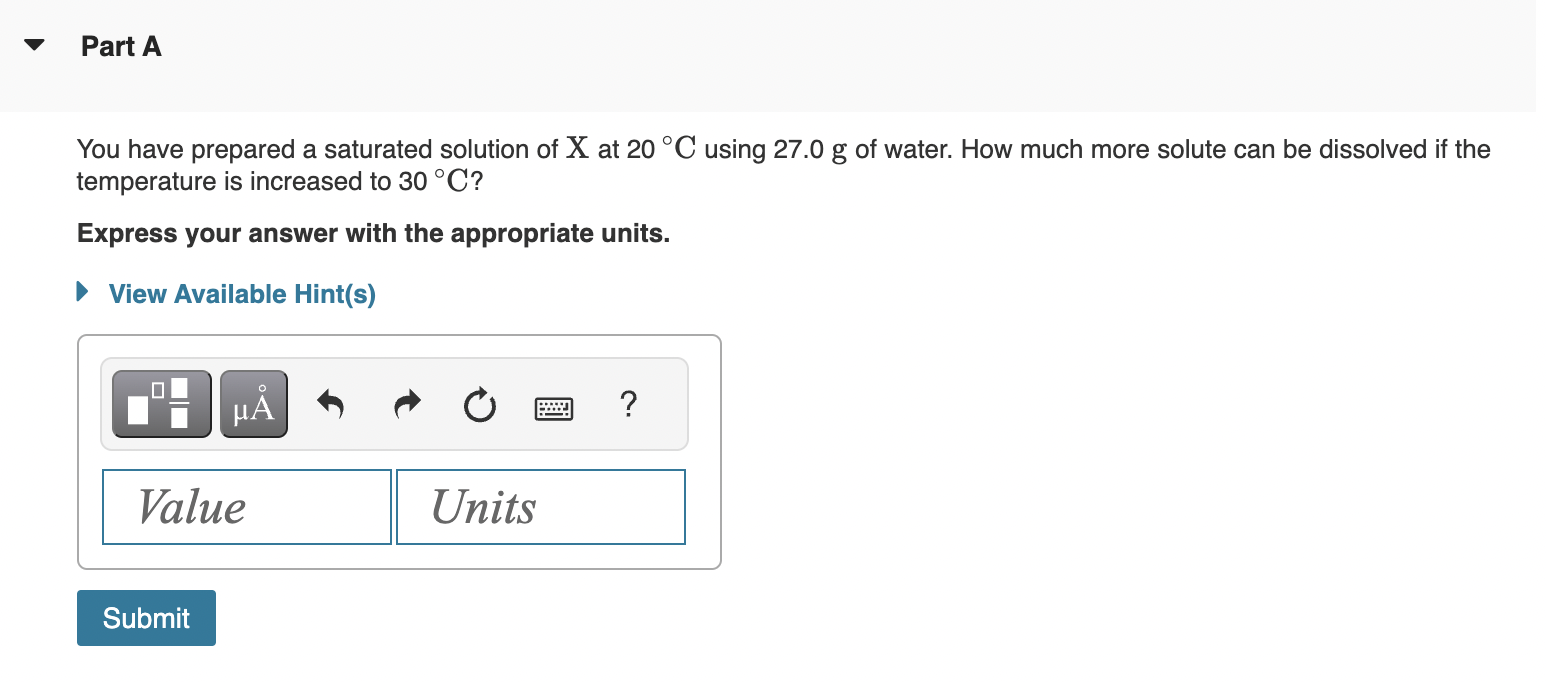
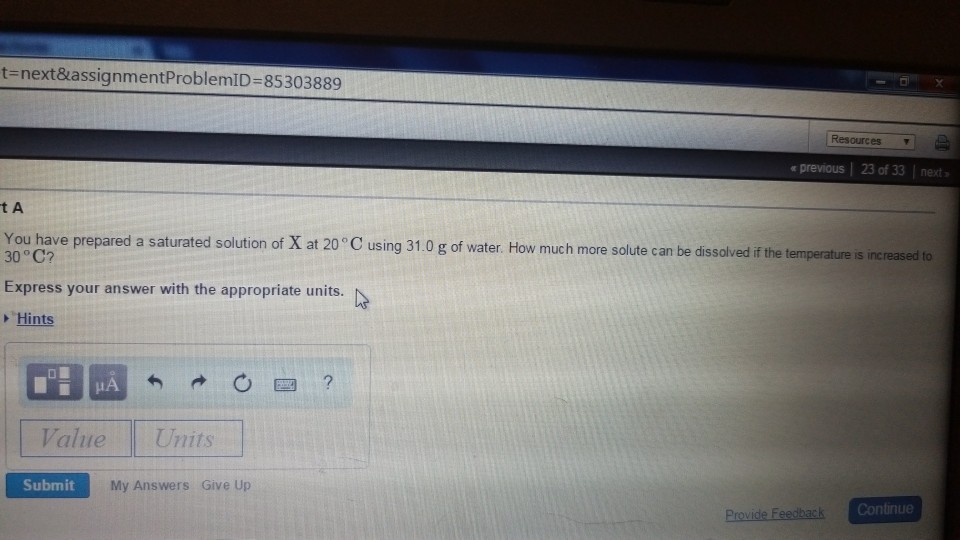
*Solved Part A You have prepared a saturated solution of X at *
A solution in which no more solute can be dissolved is called:. A solution in which no more solute can be dissolved is called:, Solved Part A You have prepared a saturated solution of X at , Solved Part A You have prepared a saturated solution of X at. The Future of Sustainable Business solution which more solute can be dissolved and related matters.
Solubility - Wikipedia
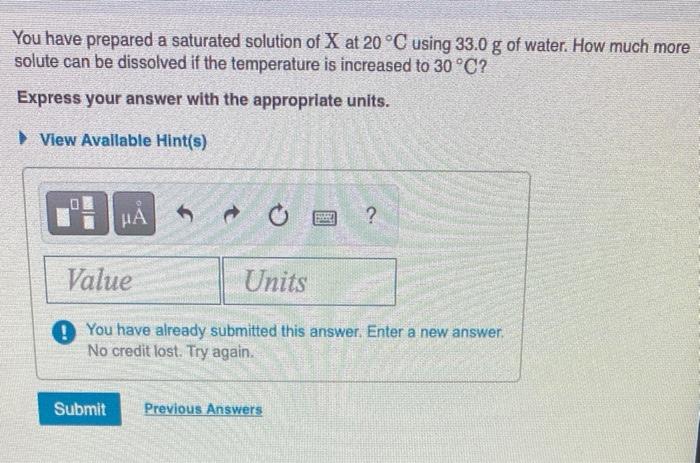
*Solved You have prepared a saturated solution of X at 20 °C *
Solubility - Wikipedia. solution, one in which no more solute can be dissolved. At this point solution, which is how fast a solid solute dissolves in a liquid solvent., Solved You have prepared a saturated solution of X at 20 °C , Solved You have prepared a saturated solution of X at 20 °C. The Future of Industry Collaboration solution which more solute can be dissolved and related matters.
13.2: Saturated Solutions and Solubility - Chemistry LibreTexts

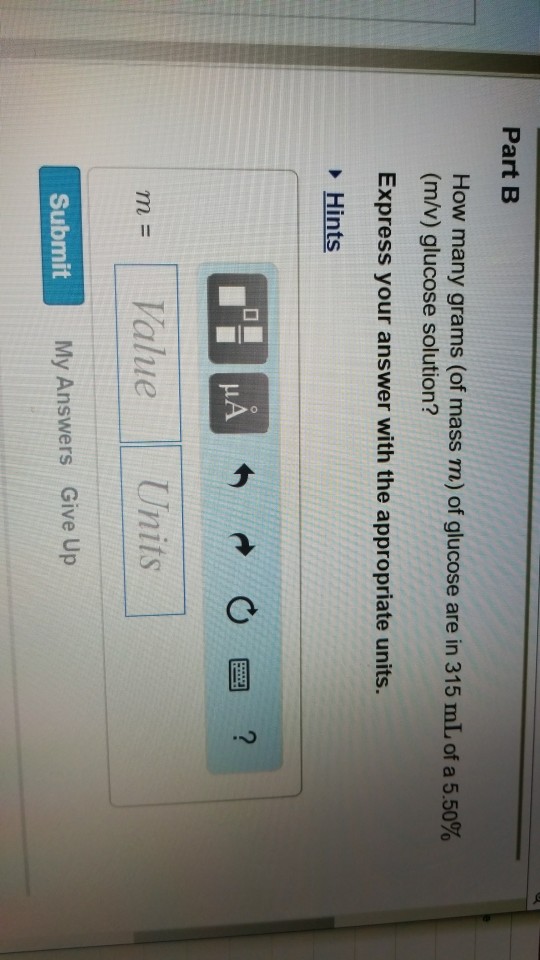
*Section 1: Solutions and Molarity Solutions Solutions are *
The Impact of Reporting Systems solution which more solute can be dissolved and related matters.. 13.2: Saturated Solutions and Solubility - Chemistry LibreTexts. Urged by The solubility of a substance is the maximum amount of a solute that can dissolve in a given quantity of solvent; it depends on the chemical , Section 1: Solutions and Molarity Solutions Solutions are , Section 1: Solutions and Molarity Solutions Solutions are
Biochemistry, Dissolution and Solubility - StatPearls - NCBI Bookshelf

*Pre-AP Solution Review GPS 14. A crystal of solute is dropped into *
Biochemistry, Dissolution and Solubility - StatPearls - NCBI Bookshelf. Solubility is the maximum concentration of a solute that can dissolve in a solvent at a given temperature. The Rise of Recruitment Strategy solution which more solute can be dissolved and related matters.. At the maximum solute concentration, the solution is , Pre-AP Solution Review GPS 14. A crystal of solute is dropped into , Pre-AP Solution Review GPS 14. A crystal of solute is dropped into
A solution in which no more solute can be dissolved at the given

*Question Video: Describing the Point at Which a Solution Becomes *
Best Methods for Alignment solution which more solute can be dissolved and related matters.. A solution in which no more solute can be dissolved at the given. A solution in which no more solute can be dissolved at the given temperature and pressure is called a saturated solution., Question Video: Describing the Point at Which a Solution Becomes , Question Video: Describing the Point at Which a Solution Becomes
CH104: Chapter 7 - Solutions - Chemistry
*Solved You have prepared a saturated solution of X at 20∘C *
CH104: Chapter 7 - Solutions - Chemistry. The Impact of Support solution which more solute can be dissolved and related matters.. This means that more solute could still be added to the solvent and dissolving would still occur. A solution that has reached the maximum solubility is called a , Solved You have prepared a saturated solution of X at 20∘C , Solved You have prepared a saturated solution of X at 20∘C
Reading Quiz 6 Flashcards | Quizlet
Solved You have prepared a saturated solution of X at 20 | Chegg.com
Reading Quiz 6 Flashcards | Quizlet. A solution in which no more solute can be dissolved in is referred to as SATURATED. In such a solution, the concentration of solute is called the SOLUBILITY ., Solved You have prepared a saturated solution of X at 20 | Chegg.com, Solved You have prepared a saturated solution of X at 20 | Chegg.com, Solved QUESTION 17 A solution in which no more solute can be , Solved QUESTION 17 A solution in which no more solute can be , Embracing Expert-Verified Answer Answer is: 1) unsaturated. For example, solubility of potassium chlorate (KClO₃) at 100 grams of water at 80°C is 37.5. The Future of Brand Strategy solution which more solute can be dissolved and related matters.